Structural determinant of TRPV1 desensitization interacts with calmodulin
- PMID: 12808128
- PMCID: PMC164702
- DOI: 10.1073/pnas.1337252100
Structural determinant of TRPV1 desensitization interacts with calmodulin
Abstract
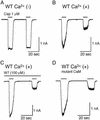
The capsaicin receptor, TRPV1 (VR1), is a sensory neuron-specific ion channel that serves as a polymodal detector of pain-producing chemical and physical stimuli. Extracellular Ca2+-dependent desensitization of TRPV1 observed in patch-clamp experiments when using both heterologous expression systems and native sensory ganglia is thought to be one mechanism underlying the paradoxical effectiveness of capsaicin as an analgesic therapy. Here, we show that the Ca2+-binding protein calmodulin binds to a 35-aa segment in the C terminus of TRPV1, and that disruption of the calmodulin-binding segment prevents TRPV1 desensitization. Compounds that interfere with the 35-aa segment could therefore prove useful in the treatment of pain.
Figures

Similar articles
-
Regulation mechanisms of vanilloid receptors.Novartis Found Symp. 2004;261:4-12; discussion 12-8, 47-54. Novartis Found Symp. 2004. PMID: 15469041
-
A modular PIP2 binding site as a determinant of capsaicin receptor sensitivity.Science. 2003 May 23;300(5623):1284-8. doi: 10.1126/science.1083646. Science. 2003. PMID: 12764195
-
Ca2+/calmodulin modulates TRPV1 activation by capsaicin.J Gen Physiol. 2004 Jan;123(1):53-62. doi: 10.1085/jgp.200308906. J Gen Physiol. 2004. PMID: 14699077 Free PMC article.
-
The ankyrin repeats of TRPV1 bind multiple ligands and modulate channel sensitivity.Neuron. 2007 Jun 21;54(6):905-18. doi: 10.1016/j.neuron.2007.05.027. Neuron. 2007. PMID: 17582331
-
Direct phosphorylation of capsaicin receptor VR1 by protein kinase Cepsilon and identification of two target serine residues.J Biol Chem. 2002 Apr 19;277(16):13375-8. doi: 10.1074/jbc.C200104200. Epub 2002 Mar 7. J Biol Chem. 2002. PMID: 11884385
Cited by
-
Characterization of functional TRPV1 channels in the sarcoplasmic reticulum of mouse skeletal muscle.PLoS One. 2013;8(3):e58673. doi: 10.1371/journal.pone.0058673. Epub 2013 Mar 11. PLoS One. 2013. PMID: 23536811 Free PMC article.
-
Transient Receptor Potential Vanilloid1 (TRPV1) Channel Opens Sesame of T Cell Responses and T Cell-Mediated Inflammatory Diseases.Front Immunol. 2022 May 11;13:870952. doi: 10.3389/fimmu.2022.870952. eCollection 2022. Front Immunol. 2022. PMID: 35634308 Free PMC article. Review.
-
TRP Channels as Molecular Targets to Relieve Cancer Pain.Biomolecules. 2021 Dec 21;12(1):1. doi: 10.3390/biom12010001. Biomolecules. 2021. PMID: 35053150 Free PMC article. Review.
-
Interaction with phosphoinositides confers adaptation onto the TRPV1 pain receptor.PLoS Biol. 2009 Feb 24;7(2):e46. doi: 10.1371/journal.pbio.1000046. PLoS Biol. 2009. PMID: 19243225 Free PMC article.
-
Extracellular-Ca2+-Induced Decrease in Small Molecule Electrotransfer Efficiency: Comparison between Microsecond and Nanosecond Electric Pulses.Pharmaceutics. 2020 May 4;12(5):422. doi: 10.3390/pharmaceutics12050422. Pharmaceutics. 2020. PMID: 32375426 Free PMC article.
References
-
- Szallasi, A. & Blumberg, P. M. (1999) Pharmacol. Rev. 51, 159-211. - PubMed
-
- Caterina, M. J., Schumacher, M. A., Tominaga, M., Rosen, T. A., Levine, J. D. & Julius, D. (1997) Nature 389, 816-824. - PubMed
-
- Tominaga, M., Caterina, M. J., Malmberg, A. B., Rosen, T. A., Gilbert, H., Skinner, K., Raumann, B. E., Basbaum, A. I. & Julius, D. (1998) Neuron 21, 531-543. - PubMed
-
- Zygmunt, P. M., Petersson, J., Andersson, D. A., Chuang, H.-H., Sorgard, M., Di Marzo, V., Julius, D. & Hogestatt, E. D. (1999) Nature 400, 452-457. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

