Pelvic Nerve Injury Causes a Rapid Decrease in Expression of Choline Acetyltransferase and Upregulation of c-Jun and ATF-3 in a Distinct Population of Sacral Preganglionic Neurons
- PMID: 21283532
- PMCID: PMC3031092
- DOI: 10.3389/fnins.2011.00006
Pelvic Nerve Injury Causes a Rapid Decrease in Expression of Choline Acetyltransferase and Upregulation of c-Jun and ATF-3 in a Distinct Population of Sacral Preganglionic Neurons
Abstract
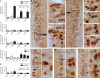
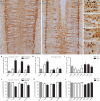
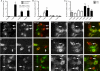
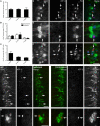
Autonomic regulation of the urogenital organs is impaired by injuries sustained during pelvic surgery or compression of lumbosacral spinal nerves (e.g., cauda equina syndrome). To understand the impact of injury on both sympathetic and parasympathetic components of this nerve supply, we performed an experimental surgical and immunohistochemical study on adult male rats, where the structure of this complex part of the nervous system has been well defined. We performed unilateral transection of pelvic or hypogastric nerves and analyzed relevant regions of lumbar and sacral spinal cord, up to 4 weeks after injury. Expression of c-Jun, the neuronal injury marker activating transcription factor-3 (ATF-3), and choline acetyltransferase (ChAT) were examined. We found little evidence for chemical or structural changes in substantial numbers of functionally related but uninjured spinal neurons (e.g., in sacral preganglionic neurons after hypogastric nerve injury), failing to support the concept of compensatory events. The effects of injury were greatest in sacral cord, ipsilateral to pelvic nerve transection. Here, around half of all preganglionic neurons expressed c-Jun within 1 week of injury, and substantial ATF-3 expression also occurred, especially in neurons with complete loss of ChAT-immunoreactivity. There did not appear to be any death of retrogradely labeled neurons, in contrast to axotomy studies performed on other regions of spinal cord or sacral ventral root avulsion models. Each of the effects we observed occurred in only a subpopulation of preganglionic neurons at that spinal level, raising the possibility that distinct functional subgroups have different susceptibility to trauma-induced degeneration and potentially different regenerative abilities. Identification of the cellular basis of these differences may provide insights into organ-specific strategies for attenuating degeneration or promoting regeneration of these circuits after trauma.
Keywords: cauda equina syndrome; inferior hypogastric plexus; micturition; regeneration; spinal cord injury; spinal nerves; sprouting.
Figures

Similar articles
-
Axonal Injury Induces ATF3 in Specific Populations of Sacral Preganglionic Neurons in Male Rats.Front Neurosci. 2018 Oct 24;12:766. doi: 10.3389/fnins.2018.00766. eCollection 2018. Front Neurosci. 2018. PMID: 30405344 Free PMC article.
-
Deafferentation and axotomy each cause neurturin-independent upregulation of c-Jun in rodent pelvic ganglia.Exp Neurol. 2009 Feb;215(2):271-80. doi: 10.1016/j.expneurol.2008.10.012. Epub 2008 Nov 6. Exp Neurol. 2009. PMID: 19038253
-
Specific targeting of ganglion cell sprouts provides an additional mechanism for restoring peripheral motor circuits in pelvic ganglia after spinal nerve damage.J Neurosci. 1998 Oct 1;18(19):7987-95. doi: 10.1523/JNEUROSCI.18-19-07987.1998. J Neurosci. 1998. PMID: 9742165 Free PMC article.
-
Segmental organization of spinal reflexes mediating autonomic dysreflexia after spinal cord injury.Prog Brain Res. 2006;152:265-74. doi: 10.1016/S0079-6123(05)52017-X. Prog Brain Res. 2006. PMID: 16198706 Free PMC article. Review.
-
Organization of lumbar spinal outflow to distal colon and pelvic organs.Physiol Rev. 1987 Oct;67(4):1332-404. doi: 10.1152/physrev.1987.67.4.1332. Physiol Rev. 1987. PMID: 2891149 Review.
Cited by
-
Identification of the Avulsion-Injured Spinal Motoneurons.J Mol Neurosci. 2015 Sep;57(1):142-51. doi: 10.1007/s12031-015-0588-4. Epub 2015 May 30. J Mol Neurosci. 2015. PMID: 26025326 Free PMC article.
-
Peripheral injury of pelvic visceral sensory nerves alters GFRα (GDNF family receptor alpha) localization in sensory and autonomic pathways of the sacral spinal cord.Front Neuroanat. 2015 Apr 10;9:43. doi: 10.3389/fnana.2015.00043. eCollection 2015. Front Neuroanat. 2015. PMID: 25914629 Free PMC article.
-
Activating transcription factor 3 and the nervous system.Front Mol Neurosci. 2012 Feb 14;5:7. doi: 10.3389/fnmol.2012.00007. eCollection 2012. Front Mol Neurosci. 2012. PMID: 22347845 Free PMC article.
-
A Novel Acute Discogenic Myelopathy Model Using Merocel® Sponge: Comparison With Clip Compression Model in Rats.Korean J Neurotrauma. 2023 Jun 23;19(2):204-217. doi: 10.13004/kjnt.2023.19.e28. eCollection 2023 Jun. Korean J Neurotrauma. 2023. PMID: 37431382 Free PMC article.
-
Axonal Injury Induces ATF3 in Specific Populations of Sacral Preganglionic Neurons in Male Rats.Front Neurosci. 2018 Oct 24;12:766. doi: 10.3389/fnins.2018.00766. eCollection 2018. Front Neurosci. 2018. PMID: 30405344 Free PMC article.
References
-
- Anderson C. R., Edwards S. L. (1994). Intraperitoneal injections of Fluorogold reliably labels all sympathetic preganglionic neurons in the rat. J. Neurosci. Methods 53, 137–141 - PubMed
-
- Armstrong D. M., Brady R., Hersh L. B., Hayes R. C., Wiley R. G. (1991). Expression of choline acetyltransferase and nerve growth factor receptor within hypoglossal motoneurons following nerve injury. J. Comp. Neurol. 304, 596–607 - PubMed
-
- Averill S., Michael G. J., Shortland P. J., Leavesley R. C., King V. R., Bradbury E. J., McMahon S. B., Priestley J. V. (2004). NGF and GDNF ameliorate the increase in ATF3 expression which occurs in dorsal root ganglion cells in response to peripheral nerve injury. Eur. J. Neurosci. 19, 1437–1445 - PubMed
-
- Barber R. P., Phelps P. E., Houser C. R., Crawford G. D., Salvaterra P. M., Vaughn J. E. (1984). The morphology and distribution of neurons containing choline acetyltransferase in the adult rat spinal cord: an immunocytochemical study. J. Comp. Neurol. 229, 329–346 - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous

