Scn2a severe hypomorphic mutation decreases excitatory synaptic input and causes autism-associated behaviors
- PMID: 34156984
- PMCID: PMC8410058
- DOI: 10.1172/jci.insight.150698
Scn2a severe hypomorphic mutation decreases excitatory synaptic input and causes autism-associated behaviors
Abstract
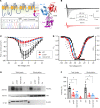
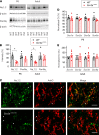
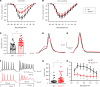
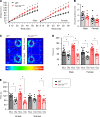
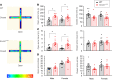
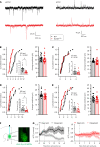
SCN2A, encoding the neuronal voltage-gated Na+ channel NaV1.2, is one of the most commonly affected loci linked to autism spectrum disorders (ASDs). Most ASD-associated mutations in SCN2A are loss-of-function mutations, but studies examining how such mutations affect neuronal function and whether Scn2a mutant mice display ASD endophenotypes have been inconsistent. We generated a protein truncation variant Scn2a mouse model (Scn2aΔ1898/+) by CRISPR that eliminates the NaV1.2 channel's distal intracellular C-terminal domain, and we analyzed the molecular and cellular consequences of this variant in a heterologous expression system, in neuronal culture, in brain slices, and in vivo. We also analyzed multiple behaviors in WT and Scn2aΔ1898/+ mice and correlated behaviors with clinical data obtained in human subjects with SCN2A variants. Expression of the NaV1.2 mutant in a heterologous expression system revealed decreased NaV1.2 channel function, and cultured pyramidal neurons isolated from Scn2aΔ1898/+ forebrain showed correspondingly reduced voltage-gated Na+ channel currents without compensation from other CNS voltage-gated Na+ channels. Na+ currents in inhibitory neurons were unaffected. Consistent with loss of voltage-gated Na+ channel currents, Scn2aΔ1898/+ pyramidal neurons displayed reduced excitability in forebrain neuronal culture and reduced excitatory synaptic input onto the pyramidal neurons in brain slices. Scn2aΔ1898/+ mice displayed several behavioral abnormalities, including abnormal social interactions that reflect behavior observed in humans with ASD and with harboring loss-of-function SCN2A variants. This model and its cellular electrophysiological characterizations provide a framework for tracing how a SCN2A loss-of-function variant leads to cellular defects that result in ASD-associated behaviors.
Keywords: Ion channels; Mouse models; Neuroscience.
Conflict of interest statement
Figures
Similar articles
-
Opposing Effects on NaV1.2 Function Underlie Differences Between SCN2A Variants Observed in Individuals With Autism Spectrum Disorder or Infantile Seizures.Biol Psychiatry. 2017 Aug 1;82(3):224-232. doi: 10.1016/j.biopsych.2017.01.009. Epub 2017 Jan 27. Biol Psychiatry. 2017. PMID: 28256214 Free PMC article.
-
Sodium Channelopathies in Human and Animal Models of Epilepsy and Neurodevelopmental Disorders.In: Noebels JL, Avoli M, Rogawski MA, Vezzani A, Delgado-Escueta AV, editors. Jasper's Basic Mechanisms of the Epilepsies. 5th edition. New York: Oxford University Press; 2024. Chapter 44. In: Noebels JL, Avoli M, Rogawski MA, Vezzani A, Delgado-Escueta AV, editors. Jasper's Basic Mechanisms of the Epilepsies. 5th edition. New York: Oxford University Press; 2024. Chapter 44. PMID: 39637100 Free Books & Documents. Review.
-
The Autism-Associated Gene Scn2a Contributes to Dendritic Excitability and Synaptic Function in the Prefrontal Cortex.Neuron. 2019 Aug 21;103(4):673-685.e5. doi: 10.1016/j.neuron.2019.05.037. Epub 2019 Jun 20. Neuron. 2019. PMID: 31230762 Free PMC article.
-
Hyperexcitability and Pharmacological Responsiveness of Cortical Neurons Derived from Human iPSCs Carrying Epilepsy-Associated Sodium Channel Nav1.2-L1342P Genetic Variant.J Neurosci. 2021 Dec 8;41(49):10194-10208. doi: 10.1523/JNEUROSCI.0564-21.2021. Epub 2021 Oct 29. J Neurosci. 2021. PMID: 34716231 Free PMC article.
-
Gene therapy as an emerging treatment for Scn2a mutation-induced autism spectrum disorders.Fundam Res. 2023 Feb 18;4(6):1401-1404. doi: 10.1016/j.fmre.2023.02.004. eCollection 2024 Nov. Fundam Res. 2023. PMID: 39734530 Free PMC article. Review.
Cited by
-
A neural perspective on the treatment of hypertension: the neurological network excitation and inhibition (E/I) imbalance in hypertension.Front Cardiovasc Med. 2024 Sep 11;11:1436059. doi: 10.3389/fcvm.2024.1436059. eCollection 2024. Front Cardiovasc Med. 2024. PMID: 39323755 Free PMC article. Review.
-
Crosstalk among WEE1 Kinase, AKT, and GSK3 in Nav1.2 Channelosome Regulation.Int J Mol Sci. 2024 Jul 24;25(15):8069. doi: 10.3390/ijms25158069. Int J Mol Sci. 2024. PMID: 39125637 Free PMC article.
-
Voltage-gated sodium channels in genetic epilepsy: up and down of excitability.J Neurochem. 2024 Dec;168(12):3872-3890. doi: 10.1111/jnc.15947. Epub 2023 Aug 31. J Neurochem. 2024. PMID: 37654020 Free PMC article. Review.
-
Cellular and behavioral effects of altered NaV1.2 sodium channel ion permeability in Scn2aK1422E mice.Hum Mol Genet. 2022 Aug 25;31(17):2964-2988. doi: 10.1093/hmg/ddac087. Hum Mol Genet. 2022. PMID: 35417922 Free PMC article.
-
Developmental and epileptic encephalopathies: from genetic heterogeneity to phenotypic continuum.Physiol Rev. 2023 Jan 1;103(1):433-513. doi: 10.1152/physrev.00063.2021. Epub 2022 Aug 11. Physiol Rev. 2023. PMID: 35951482 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

